AFBAU PRINCIPLE
The aufbau principle states that in the ground state of an atom or ion, electrons fill atomic orbitals of the lowest available energy levels before occupying higher levels. For example, the 1s shell is filled before the 2s subshell is occupied. In this way, the electrons of an atom or ion form the most stable electron configuration possible. An example is the configuration 1s2 2s2 2p6 3s2 3p3 for the phosphorus atom, meaning that the 1s subshell has 2 electrons etc.
Aufbau is a German noun that means construction or "building-up". The aufbau principle is sometimes called the building-up principle or the aufbau rule.
The details of this "building-up" tendency are described mathematically by atomic orbital functions. Electron behavior is elaborated by other principles of atomic physics, such as Hund's rule and the Pauli exclusion principle. Hund's rule asserts that if multiple orbitals of the same energy are available, electrons will occupy different orbitals singly before any are occupied doubly. If double occupation does occur, the Pauli exclusion principle requires that electrons which occupy the same orbital must have different spins (+1/2 and −1/2).
As we pass from one element to another of next higher atomic number, one proton and one electron are added each time to the neutral atom. The maximum number of electrons in any shell is 2n2, where n is the principal quantum number. The maximum number of electrons in a subshell (s, p, d or f) is equal to 2(2ℓ+1) where ℓ = 0, 1, 2, 3... Thus these subshells can have a maximum of 2, 6, 10 and 14 electrons respectively. In the ground state the electronic configuration can be built up by placing electrons in the lowest available orbitals until the total number of electrons added is equal to the atomic number. Thus orbitals are filled in the order of increasing energy, using two general rules to help predict electronic configurations:
- 1. Electrons are assigned to orbitals in order of increasing value of (n+ℓ).
- 2. For subshells with the same value of (n+ℓ), electrons are assigned first to the sub shell with lower n.
A version of the aufbau principle known as the nuclear shell model is used to predict the configuration of protons and neutrons in an atomic nucleus.[1]
Contents
Madelung energy ordering rule[edit]
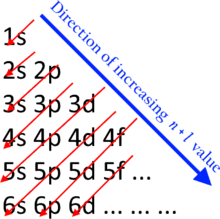
In neutral atoms, the order in which subshells are filled is given by the n + ℓ rule, also known as the Madelung rule (after Erwin Madelung), or the Janet rule or the Klechkowsky rule (after Charles Janet or Vsevolod Klechkovsky in some, mostly French and Russian-speaking, countries), or the diagonal rule.[2] Orbitals with a lower n + ℓ value are filled before those with higher n + ℓ values. In this context, nrepresents the principal quantum number and ℓ the azimuthal quantum number; the values ℓ = 0, 1, 2, 3 correspond to the s, p, d, and flabels, respectively. The subshell ordering by this rule is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, 8s, ... For example titanium (Z = 22) has the ground-state configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d2 [3]
Other authors write the orbitals always in order of increasing n, such as Ti (Z = 22) 1s2 2s2 2p6 3s2 3p6 3d2 4s2.[4] This can be called "leaving order", since if the atom is ionized, electrons leave in the order 4s, 3d, 3p, 3s, etc. For a given neutral atom, the two notations are equivalent since only the orbital occupancies have physical significance.
The rule is based on the total number of nodes in the atomic orbital, n + ℓ, which is related to the energy.[5] In the case of equal n + ℓ values, the orbital with a lower n value is filled first. The fact that most of the ground state configurations of neutral atoms fill orbitals following this n + ℓ, n pattern was obtained experimentally, by reference to the spectroscopic characteristics of the elements.[6]
The Madelung energy ordering rule applies only to neutral atoms in their ground state. There are 10 elements among the transition metalsand 9 elements among the lanthanides and actinides for which the Madelung rule predicts an electron configuration that differs from that determined experimentally by one electron orbit (in the case of palladium and thorium by two electron orbits).[7]
Exceptions to the Madelung rule in the transition metals:
The valence d-subshell "borrows" one electron (in the case of palladium two electrons) from the valence s-subshell.
| Atom | 24Cr | 29Cu | 41Nb | 42Mo | 44Ru | 45Rh | 46Pd | 47Ag | 78Pt | 79Au |
|---|---|---|---|---|---|---|---|---|---|---|
| Core electrons | [Ar] | [Ar] | [Kr] | [Kr] | [Kr] | [Kr] | [Kr] | [Kr] | [Xe] | [Xe] |
| Madelung Rule | 3d44s2 | 3d94s2 | 4d35s2 | 4d45s2 | 4d65s2 | 4d75s2 | 4d85s2 | 4d95s2 | 4f145d86s2 | 4f145d96s2 |
| Experiment | 3d54s1 | 3d104s1 | 4d45s1 | 4d55s1 | 4d75s1 | 4d85s1 | 4d10 | 4d105s1 | 4f145d96s1 | 4f145d106s1 |
Example copper 29Cu: According to the Madelung rule, the 4s orbital (n + ℓ = 4 + 0 = 4) is occupied before the 3d orbital (n + ℓ = 3 + 2 = 5). The rule then predicts the electron configuration 1s22s22p63s2 3p63d94s2, abbreviated [Ar]3d94s2 where [Ar] denotes the configuration of the preceding noble gas argon. However the measured electron configuration of the copper atom is [Ar]3d104s1. By filling the 3d orbital, copper can be in a lower energy state.
Exceptions to the Madelung rule in the lanthanides and actinides:
The valence d-subshell "borrows" one electron (in the case of thorium two electrons) from the valence f-subshell.
| Atom | 57La | 58Ce | 64Gd | 89Ac | 90Th | 91Pa | 92U | 93Np | 96Cm |
|---|---|---|---|---|---|---|---|---|---|
| Core electrons | [Xe] | [Xe] | [Xe] | [Rn] | [Rn] | [Rn] | [Rn] | [Rn] | [Rn] |
| Madelung Rule | 4f16s2 | 4f26s2 | 4f86s2 | 5f17s2 | 5f27s2 | 5f37s2 | 5f47s2 | 5f57s2 | 5f87s2 |
| Experiment | 5d16s2 | 4f15d16s2 | 4f75d16s2 | 6d17s2 | 6d27s2 | 5f26d17s2 | 5f36d17s2 | 5f46d17s2 | 5f76d17s2 |
Example uranium 92U: According to the Madelung rule, the 5f orbital (n + ℓ = 5 + 3 = 8) is occupied before the 6d orbital (n + ℓ = 6 + 2 = 8). The rule then predicts the electron configuration [Rn]5f47s2 where [Rn] denotes the configuration of the preceding noble gas radon. However the measured electron configuration of the uranium atom is [Rn]5f36d17s2.
A special exception is lawrencium 103Lr, where the 6d electron predicted by the Madelung rule is replaced by a 7p electron: the rule predicts [Rn]5f146d17s2, but the measured configuration is [Rn]5f147s27p1.
| Atom | 103Lr |
|---|---|
| Core electrons | [Rn] |
| Madelung Rule | 5f146d17s2 |
| Experiment | 5f147s27p1 |
History[edit]
The aufbau principle in the new quantum theory[edit]
The principle takes its name from the German, Aufbauprinzip, "building-up principle", rather than being named for a scientist. In fact, it was formulated by Niels Bohr and Wolfgang Pauli in the early 1920s, and states that:
| “ | The orbitals of lower energy are filled in first with the electrons and only then the orbitals of high energy are filled. | ” |
This was an early application of quantum mechanics to the properties of electrons, and explained chemical properties in physical terms. Each added electron is subject to the electric field created by the positive charge of the atomic nucleus and the negative charge of other electrons that are bound to the nucleus. Although in hydrogen there is no energy difference between orbitals with the same principal quantum number n, this is not true for the outer electrons of other atoms.
In the old quantum theory prior to quantum mechanics, electrons were supposed to occupy classical elliptical orbits. The orbits with the highest angular momentum are 'circular orbits' outside the inner electrons, but orbits with low angular momentum (s- and p-orbitals) have high orbital eccentricity, so that they get closer to the nucleus and feel on average a less strongly screened nuclear charge.
The n + ℓ energy ordering rule[edit]
A periodic table in which each row corresponds to one value of n + ℓ was suggested by Charles Janet in 1928, and in 1930 he made explicit the quantum basis of this pattern, based on knowledge of atomic ground states determined by the analysis of atomic spectra. In 1936, the German physicist Erwin Madelung proposed this as an empirical rule for the order of filling atomic subshells, and most English-language sources therefore refer to the Madelung rule. Madelung may have been aware of this pattern as early as 1926.[8] In 1962 the Russian agricultural chemist V.M. Klechkowski proposed the first theoretical explanation for the importance of the sum n + ℓ, based on the statistical Thomas–Fermi model of the atom.[9] Many French- and Russian-language sources therefore refer to the Klechkowski rule.
In recent years it has been noted that the order of filling orbitals in neutral atoms does not always correspond to the order of adding or removing electrons for a given atom. For example, in the fourth row of the periodic table the Madelung rule indicates that the 4s orbital is occupied before the 3d. The neutral atom ground state configurations are therefore K = (Ar)4s, Ca = (Ar)4s2, Sc = (Ar)4s23d, etc. However if a scandium atom is ionized by removing electrons (only), the configurations are Sc = (Ar)4s23d, Sc+ = (Ar)4s3d, Sc2+ = (Ar)3d. The orbital energies and their order depend on the nuclear charge; 4s is lower than 3d as per the Madelung rule in K with 19 protons, but 3d is lower in Sc2+ with 21 protons. The Madelung rule should only be used for neutral atoms.
In addition to there being ample experimental evidence to support this view, it makes the explanation of the order of ionization of electrons in this and other transition metals far more intelligible, given that 4s electrons are invariably preferentially ionized.[10]

Comments
Post a Comment